ABSTRACT
A fungal infection was found in eggs and larvae of black tiger shrimp Penaeusmonodon at a hatchery in Chachensao Province, Thailand in August 2000. Fungi were isolatedfrom eggs and larvae with fungal infection, and studied on the morphological and biological charac-teristics. When it was transferred from PYGS broth to artificial seawater, discharge tubes devel-oped from the mycelia, and a vesicle for zoospore formation was produced at the top of eachdischarge tube. The characteristic feature of an asexual reproduction of the fungus was thatzoospores swam away in seawater after the vesicle separated from the discharge tube. Based onthese morphological characteristics, the fungus was identified as Lagenidium thermophilum.Some biological characteristics of the selected isolate NJM 0031 were compared with the otherspecies in the genus Lagenidium isolated from some crustaceans. As a result, the isolate NJM0031 showed similar characteristics to those of L. thermophilum ATCC 200318 isolated from man-grove crab Scylla serrata. The isolate was demonstrated to be pathogenic to larvae of black tigershrimp by artificial infection. This is the first report of L. thermophilum infection in black tigershrimp in Thailand.
Larvae of black tiger shrimp Penaeus monodon areproduced at many hatcheries in Chachensao Province,Thailand. Fungal infection has been often found in theireggs and larvae. When the infection occurred at thesehatcheries, it has been known as a problematic infection,because it caused high mortality in the larvae. Thereare no scientific reports on fungal infections of black tigershrimp in Thailand.
In August 2000, a fungal infection occurred in eggsand larvae of black tiger shrimp P. monodon at a hatch-ery in Chachensao Province, Thailand. This paperdescribes the identification of the causative fungus iso-lated from these eggs and larvae.
Materials and Methods
Isolation and identification
Eggs (Fig. 1) and zoeae (Fig. 2) of black tigershrimp P. monodon with a fungal infection were directlyinoculated on PYGS agar [0.125% peptone, 0.125%yeast-extract, 0.3% glucose, 1.2% agar, and 37.6 g ofartificial seawater (Aqua-Ocean®, Japan Pet Drugs,Tokyo, Japan)] plates, and then streptomycin sulfate andampicillin were added on the medium for reduction ofbacterial growth and incubated at 25oC. Each fungalcolony was transferred onto new PYGS agar to make apure culture. As all isolates were judged as the samefungus, an isolate NJM 0031 was randomly selectedfrom the isolates, and used for all the followingexperiments. For morphological observations, the iso-late was cultured in PYGS broth at 25oC for 4 days, anda part of mycelia in PYGS broth were rinsed with steril-ized artificial seawater, then transferred into sterilizedartificial seawater and incubated at 25oC for 36 h. Thefungus was identified according to Sparrow (1960),Bian et al. (1979) and Karling (1981).Lagenidiumcallinectes ATCC 24973, L. callinectes NJM 8989, L.thermophilum ATCC 200318 and L. myophilum ATCC66280 known as pathogens of various crustaceans wereused for comparisons in biological and physiologicalexperiments (Table 1).
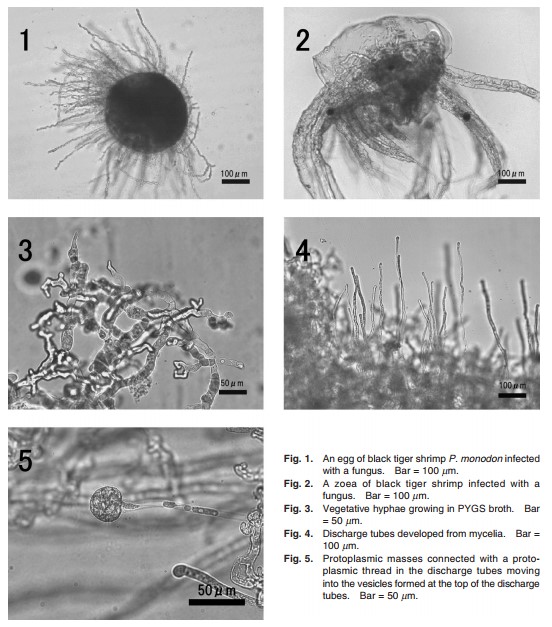
Table 1. Source of Lagenidium strains used in this study
| Species | Strains | Locality | Host |
Year |
|
L. thermophilum |
NJM 0031 | Chachensao, Thailand | P. monodon | 2000 |
|
L. thermophilum |
NJM 9338 (ATTCb 200318) | Bali, Indonesia | Scylla serrata | 1993 |
|
L. Callinectes |
ATCC 24973 | North Carolina, U. S. A | Callinectes sapidus |
1973 |
| L. Callinectes | NJM 8989 | Okayama, Japan | Portunus trituberculatus |
1986 |
| L. myophilum | NJM 8601 (ATCC 66280) | Ishikawa, Japan | Pandalus borealis |
1988 |
a Culture collection in the Division of Fish Diseases, Nippon Veterinary and Animal Science University, Musashino,Tokyo, Japan.
b American Type Culture of Collection, Manassas, VA, U. S. A
Effect of temperature on growth
The isolate NJM 0031, Lagenidium callinectesATCC 24973, L. callinectes NJM 8989, L. thermophilumATCC 200318 and L. myophilum ATCC 66280 wereinoculated onto PYGS agar plate and incubated at 25oCfor 10 days to allow to form a large colony. Agar blockstaken from the edge of a growing colony with a No. 2cork borer (5.5 mm diam.) were inoculated onto the cen-ter of PYGS agar plates. Each plate was incubated atnine different temperatures (5, 10, 15, 20, 25, 30, 35, 40, 45oC). The colony radius was measured every 2 daysfor 10 days after the inoculation
Effect of NaCl concentration on growth
The same five isolates were cultured on PYGS agarplates and a part of a growing colony was inoculated aspreviously onto the center of PYG agar plates (PYGSagar without artificial seawater) containing various con-centrations of NaCl (0, 0.5, 1.0, 2.0, 3.0, 5.0%) and incu-bated at optimum temperatures for each isolate. Thecolony radius was measured every 2 days for 14 daysafter inoculation.
Experimental infection
The pathogenicity of the isolate NJM 0031 to zoeaeand mysis of black tiger shrimp was estimated by expos-ing zoospores. Twenty larvae were put into a plasticpetri dish with 30 mL seawater. The number ofzoospores in the seawater was adjusted to 1.0 × 104, 1.0 × 103 and 1.0 × 102 zoospores/mL. In a control group,the petri dish with 30 mL seawater had only 20 larvae.To reduce bacterial contaminants, each 200 µg/mL ofstreptomycin sulfate and ampicillin was added in theseawater. Each larva was examined under an invertedmicroscope at every 24 h for 2 days after exposing tozoospores. The experiment was conducted at 30oC without feeding.
Results
Identification
The present isolate NJM 0031 was whitish, flat andfilamentous on PYGS agar, and the vegetative hyphaewere non-septate with numerous protoplasmic oil drop-lets, irregularly branched, and 15–30 µm in width inPYGS broth (Fig. 3). Zoospore formation was observedat about 36 h after the mycelia were transferred into ster-ilized artificial seawater. In the process of zoospore for-mation, discharge tubes were developed gradually fromhyphae (Fig. 4), and protoplasmic masses with numer-ous oil droplets moved into the vesicles which wereformed at the top of the discharge tubes (Fig. 5). Dis-charge tubes were straight, 7–10 µm in diameter. Eachprotoplasmic mass was connected with a protoplasmicthread. After masses of all protoplasm had entered intothe vesicle, it was divided into zoospores completelywithin 15 min, swimming zoospores were observed atabout 25 min, and zoospore liberation occurred at about 30 min. Before zoospores liberate, the vesicle sepa-rated from the discharge tube (Fig. 6a–d). Afterzoospores were liberated, the vesicle was not persistent. Matured vesicles were globose to subglobose, 30–40 µm in diamter. Zoospores were laterally biflagellated, pyriform to subglobose, monoplanetic, 13 × 9 µm onaverage (Fig. 7). After swimming for several hours, zoospores were encysted. Encysted zoospores wereglobose without flagella, 10 µm in diamter on average (Fig. 8). No sexual organs were observed.
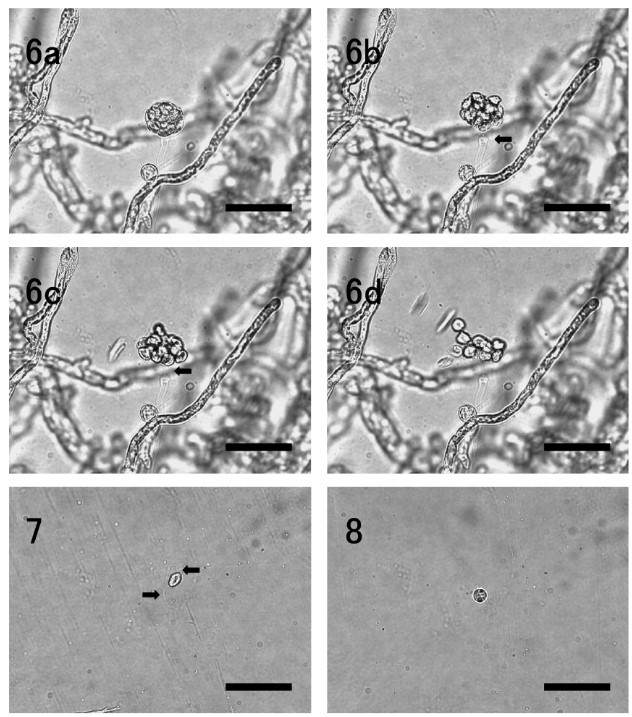
Fig. 6. Zoospores liberation of L. thermophilum NJM 0031. a, vesicle formed at the top of the discharge tube; b, zoospore liberationoccurred after the vesicle was separated from the discharge tube (arrow); c–d, all zoospores swum away simultaneously when the vesicle was burst (arrow); d, vesicle was not persistent. Bar = 50 µm
Fig. 7. A swimming zoospore. Arrows show lateral biflagellates. Bar = 50 µm.
Fig. 8. An encysted zoospore. Bar = 50 µm.
Effect of temperature on growth
The results are shown in Table 2. The isolate NJM0031 grew at 15 to 35oC with the optimum temperatureat 30oC.L. callinectes ATCC 24973 grew at 15 to 25oC, best at 25oC.L. callinectes NJM 8989 grew at 15to 25oC, best at 20oC.L. thermophilum ATCC 200318grew at 20 to 40oC, best at 30oC. L.thermophilum ATCC ATCC66280 grew at 5 to 30oC, best at 15 to 30oC. The tem-perature for the growth of the isolate NJM 0031 was almost similar to that of L.thermophilum ATCC ATCC 200318.
Effect of NaCl on growth
The results are shown in Table 3. The isolate NJM0031, L. thermophilum ATCC 200318 and L. myophilumATCC 66280 could grow on PYG agar containing 0 to5.0% NaCl.L. callinectes ATCC 24973 and L.callinectes NJM 8989 could grow on PYG agar contain-ing 0.5 to 5.0% NaCl. The growth of the isolate NJM0031 and L .thermophilum ATCC 200318 were almostthe same, irrespective of the NaCl concentration
Experimental infection
The isolate NJM 0031 showed pathogenicity tozoeae and mysis of black tiger shrimp (Table 4). Thicknon-septate hyphae were observed in the dead zoeaeand mysis under a microscope. The cumulative mor-talities in zoeae and mysis exposed to 104 zoospores/mLwere approximately 40 and 85%, respectively, at 48 hpost-exposure. In control, no infection occurred in bothzoeae and mysis.
Table 4. Pathogenicity of the isolate NJM 0031 to zoeae andmysis of black tiger shrimp by artificial infection
| No. of zoosporeszoea mysischallenged (spore/mL) | Zoea |
Mysis |
||
| 24a | 48 | 24 | 48 | |
| 10×104 | 20b | 40 | 40 | 85 |
| 10×103 | 5 | 5 | 30 | 45 |
| 10×102 | 0 | 0 | 5 | 5 |
| Đối chứng | 0 | 0 | 0 | 0 |
a : Hours after challenge.
b : Cumulative mortality (%).
Discussion
At present, the fungi in the four genera, Lagenidium,Haliphthoros, Halocrusticida and Atkinsiella, belongingto class Peronosporomycetes (Oomycetes) are knownas pathogens in marine crustaceans (Roza andHatai, 1999, Hatai et al., 2000). The classPeronosporomycetes is now transferred from thekingdom Fungi to the newly constructed kingdomStramenopila (Chromista) based on molecular phyloge-netic analysis. However, the infection caused by some members of Peronosporomycetes in marine crustaceansis still classified as fungal infection in the field of shellfishdisease.
The isolate NJM 0031 with laterally biflagellatezoospores was endobiotic and holocarpic, and producedvesicles on the top of discharge tubes. Therefore, thefungus was classified into the genus Lagenidium (Lagenidiales, Peronosporomycetes) based on thesemorphological characteristics. Fungi in the genusLagenidium have been isolated from various aquatic ani-mals (Table 5). Zoospore liberation of the isolate NJM0031 characteristically occurred after the vesicle wasseparated from the discharge tubes (Fig. 6a-d). Basedon this manner of zoospore liberation, the isolate NJM0031 was identified as L. thermophilum reported byNakamura et al. (1995). The optimum temperature forthe growth of the fungus and the isolate L. thermophilumATCC 200318 was similar.
In this study L. thermophilum ATCC 200318 could grow at 20–40oC. Nakamura et al. (1995), however, reported thatL. thermophilum ATCC 200318 could grow at 15–45oCwith an optimum of 30–40oC.L. callinectes ATCC24973 and NJM 8989 could grow at 15–25oC with anoptimum of 20oC.L. myophilum ATCC 66280 evengrew at 5oC. As a result, it was thought that theseresults were related closely to the climate where eachstrain was isolated. The isolate NJM 0031 and L. thermophilum ATCC 200318 grew on PYG agar contain-ing 0–5.0% NaCl, but poorly at 0% NaCl.L. myophilumATCC 66280 also could grow on PYG agar containing0–5.0% NaCl, but the growth was obviously differentfrom the isolate NJM 0031 and L. thermophilum ATCC200318. It grew rapidly on all salinity ranges of PYGagars. As the isolates ATCC 24973 and NJM 8989 ofL. callinectes could not grow on PYG agar containing 0%NaCl, it was thought that they were obligate marinefungi. The artificial infection demonstrated that the iso-late NJM 0031 was pathogenic to zoeae and mysis ofblack tiger shrimp. Pathogenicity of the isolate NJM0031 to mysis was higher than that to zoeae. Nakamura et al. (1995) also reported that L. thermophilum showed the pathogenicity to zoeae ofswimming crab Portunus trituberculatus by artificialinfection
Bảng 5. Nấm trong chi Lagenidium đã được báo cáo trước đây là mầm bệnh cho động vật thủy sinh.
|
Species |
Reference | Host |
Stage
|
| 1. L. giganteum | Couch (1935) | mosquito
daphnia, copepods |
larvae
– – |
| 2. L. Callinectes | Couch (1942) |
blue crab Callinectes sapidus
|
eggs |
| 3. L. scyllae | Bian et al. (1979) |
mangrove crab Scylla serrata
|
eggs and larvae
|
| 4. L.myophilum | Hatai and Lawhavinit (1988)
Nakamura et al. (1994) |
northern shrimp Pandalus borealis
coonstripe shrimp Pandalus hypsinotus |
adults
adults |
| 5. L. thermophilum | Nakamura et al. (1995) | mangrove crab Scylla serrata | eggs and larvae |
| 6. L.sp. | Lightner và Fontaine (1973) | Tôm thẻ trắng Đại Tây Dương Penaeus setiferus | eggs and larvae |
Four species in the genus Lagenidium have beenreported as pathogens of some crustaceans.L.callinectes was first reported as a pathogenic fungus ofeggs of the blue crab Callinectes sapidus (Couch, 1942),and later found in eggs and larvae of various crabs andshrimps (Crisp et al., 1989).L. scyllae was reported asa parasite of eggs and larvae of mangrove crab Scyllaserrata in Philippines (Bian et al., 1979).L. myophilumwas first described as a pathogen of northern shrimpPandalus borealis in Japan (Hatai and Lawhavinit,1988), and also from coonstripe shrimp Pandalushypsinotus (Nakamura et al., 1994).L. thermophilumwas first described as a pathogen of eggs and larvae ofmangrove crab Scylla serrata in Indonesia (Nakamura etal., 1995). The main two characteristics of L.thermophilum reported by Nakamura et al. (1995) wereas follows; the vesicle was separated from the dischargetubes before zoospores were liberated, and the optimumtemperature for growth was 30oC. The present isolateNJM 0031 also had the same characteristics when com-pared with L. thermophilum ATCC 200318 reported byNakamura et al. (1995). The species has been isolatedfrom Thailand and Indonesia. It means this species iswell adapted to the tropical regions. This is the firstreport of isolation of L. thermophilum from black tigershrimp P. monodon in Thailand
By Kishio Hatai
“Domesticated Shrimp Postlarvae – The Key To Success”
See more:
- International Artemia Aquaculture Consortium Plans To Launch This Year
- Pacific White Shrimp Nursery Trials In Seawater And Low-Salinity Water Utilizing A Synbiotic System
- Black Tiger Shrimp Processing Waste Can Be Converted Into A Value-Added Powder

 Tiếng Việt
Tiếng Việt 中文 (中国)
中文 (中国)