Abstract
In this study, vegetative cell suspensions of two Bacillus subtilis strains, L10 and G1 in equal proportions, was administered at two different doses 105 (BM5) and 108 (BM8) CFU ml-1 in the rearing water of shrimp (Litopenaeus vannamei) for eight weeks. Both probiotic groups showed a significant reduction of ammonia, nitrite and nitrate ions under in vitro and in vivo conditions. In comparison to untreated control group, final weight, weight gain, specific growth rate (SGR), food conversion ratio (FCR) and digestive enzymatic activity were significantly greater in the BM5 and BM8 groups. Significant differences for survival were recorded in the BM8 group as compared to the control. Eight weeks after the start of experiment, shrimp were challenged with Vibrio harveyi. Statistical analysis revealed significant differences in shrimp survival between probiotic and control groups. Cumulative mortality of the control group was 80%, whereas cumulative mortality of the shrimp that had been given probiotics was 36.7% with MB8 and 50% with MB5. Subsequently, real-time RT-PCR was employed to determine the mRNA levels of prophenoloxidase (proPO), peroxinectin (PE), lipopolysaccharide- and β-1,3-glucan- binding protein (LGBP) and serine protein (SP). The expression of all immune-related genes studied was only significantly up-regulated in the BM5 group compared to the BM8 and control groups. These results suggest that administration of B. subtilis strains in the rearing water confers beneficial effects for shrimp aquaculture, considering water quality, growth performance, digestive enzymatic activity, immune response and disease resistance.
Keywords:
Aquaculture; Litopenaeus vannameiBacillus subtilisVibriosisImmune response
1/ Introduction
Shrimp production has rapidly expanded with the development and intensification of aquaculture systems; however, this successful industry has been threatened by diseases caused, mainly, by opportunistic pathogens, such as Vibrio species.
The use of probiotics has been recommended as an alternative to antibiotic application due to the emergence of antibioticresistant bacteria. Different aspects of probiotics for shrimp culture have been studied and very promising results have been obtained. For instance, dietary administration of Pediococcus acidilactici resulted in an improved survival rate in shrimp, Litopenaeus stylirostris.
Previous studies have also demonstrated that Bacillus subtilis strains may be used as potential probiotics for shrimp aquaculture. In fact, Liu et al. demonstrated that white shrimp, Litopenaeus vannamei, fed a diet containing B. subtilis E20 has better growth performance than the control group. Subsequently, Liu et al. showed that the application of this strain as water additives improved the survival rate and immune response in white shrimp larvae. Similar results were reported by Shen et al. who observed that white shrimp fed a diet containing B. subtilis has better growth performance, immune response and antioxidant activity than the control group.
It is important to mention that the shrimp defence mechanisms are largely dependent on the innate immune system, and in fact is incapable of responding to specific vaccines. Thus, the use of B. subtilis as a promising probiotic may compensate the vaccine limitations for shrimp aquaculture. In our previous study, we reported that white shrimp fed a diet containing B. subtilis L10 and G1 had a higher immune gene expression than the control. A higher survival rate was also observed in treated shrimp than the control group, after they were experimentally infected with Vibrio harveyi.
Optimal environmental condition and the physico-chemical status of the rearing water is an important concern in shrimp aquaculture. Persistent or recurrent infections may be due to poor water quality and low water exchange rates. Considering culture conditions, bacteria with probiotic properties can be helpful in terms of shrimp health management; however, little information is available regarding the bioremediation effects of probiotics. Previous studies have demonstrated that several B. subtilis strains produce a variety of extracellular enzymes and antimicrobial peptides. The secretion of these substances not only is documented for controlling pathogenic bacteria but also may be beneficial for the improvement of the rearing water quality.
A potential probiotic strain needs a thorough evaluation of the required probiotic characteristics and appropriate safety assessment, prior to commercialization. We previously reported the isolation, identification, characterization, and safety of two B. subtilis L10 and G1 with antagonistic ability against two shrimp pathogens, V. harveyi and Vibrio parahaemolyticus. In addition we recently achieved promising results in white shrimp by dietary administration of these two strains. Consequently, the aims of the current study were to investigate the effects of probiotic administration, containing B. subtilis L10 and G1 at two different doses, in the rearing water on water quality, growth performance, digestive enzymatic activity, immune gene expression and disease resistance of juvenile white shrimp
2/ Materials and methods
2.1. Bacterial strains
Bacillus subtilis L10 and G1, previously isolated and identified from fermented pickles, were used as potential probiotics. A shrimp pathogen, V. harveyi ATCC 14126, was used for experimental infection. All strains were kept at -20°C in LuriaeBertani broth (LB; Difco) containing 15% glycerol (v/v) until use.
2.2. In vitro assays on the ion concentrations
Effect of two B. subtilis strains on the ion concentrations was determined using synthetic pond water according to Lalloo et al. [13] with slight modifications. Briefly, synthetic pond water was prepared using 0.0085% m/v KNO3, 0.006% m/v NaNO2, 0.0093% m/ v (NH4)2SO4, 1% m/v NaCl2, 0.1 m/v yeast extract, and 0.1% m/v glucose, with a pH adjusted to 7.0 and sterilization through 0.22 mm sterile filter.
A volume of 5 ml of each strain, previously grown overnight, was added to a 1000-ml Erlenmeyer containing 500 ml of synthetic pond water. Flasks were then incubated at 30°C on a rotary shaker at 150 rpm min-1. Each flask was aseptically sampled prior to inoculation and at 2, 8, 16, and 24 h. Nitrite, nitrate, and ammonia concentrations were assessed spectrophotometrically using HACH kits according to manufacturer’s instructions (HACH Company; Loveland, CO, USA). The decrease in ion concentrations was expressed as mg l-1 h-1. A control without addition of probiotics was included. The growth of both strains in the synthetic water was also spectrophotometrically monitored by measuring optical density at 600 nm (OD600) after 0, 2, 8, 16, and 24 h. All assays were conducted in triplicate.
2.3. Probiotic preparation
Two B. subtilis strains were grown in LB broth using a shaking incubator at 30°C for 48 h. The cultures were then centrifuged at 3000 g for 10 min at 4°C and after discarding the supernatant, the pelleted bacteria were re-suspended and washed three times in sterile normal saline solution (NSS, 0.9% NaCl). The density of cell suspensions was calculated using a spectrophotometer at 600 nm and also correlated to colony-forming units (CFU) using a spreadplate technique. These vegetative cell suspensions were kept at 4°C and immediately carried to the shrimp hatchery and added to the rearing tanks.
2.4. Shrimp and experimental conditions
Healthy juvenile shrimp were provided by the Marine Science Research Station and Biology Field Station, UPM, Port Dickson, Malaysia and the experiment was conducted at the same place. The shrimp had not been exposed to shrimp diseases and were deemed pathogen-free by standard molecular techniques. The shrimp were acclimatized for 1-week in tanks before the start of the trial. After the acclimation period, the average weigh of the shrimp was 0.67g, and the shrimp were randomly distributed into six 500-l tanks, each containing 100 shrimp. All shrimp were fed a commercial diet (BLANCA, Malaysia) three times daily (8:00 am, 16:00 pm, and 24:00 pm) at 5% body weight. The experimental units were maintained under constant aeration (5 ± 0.5% dissolved oxygen), with a 50% water change twice a week, ambient temperature of 28 ± 1 °C and pH of 7.3 – 8.2. Temperature, dissolved oxygen and pH were measured using a YSI multiprobe water quality system (Yellow Spring Inc.). Water chemical parameters, such as ammonia, nitrite and nitrate were measured spectrophotometrically once a week using HACH kits as mentioned above.
Vegetative cell suspensions of B. subtilis strains, L10 and G1 in equal proportions, were added into the rearing water to give a final concentration of approximately 105 CFU ml-1(or BM5), and 108 CFU ml-1 (or BM8). The lower concentration was selected based on previous studies, whereas the higher concentration was chosen as it is often considered that higher concentrations are more effective. A third group without probiotics was the control. All experiments were conducted in duplicate for 8 weeks and the probiotics were added to the rearing water twice a week
At the end of the experiment, the final weight, survival rate, weight gain, feed conversion ratio (FCR), and specific growth rate (SGR) of different treatments were calculated according to previously described methods. Five shrimp from each replicate was randomly collected at week 0, 4, and 8 of the experiment to estimate Bacillus sp. and Vibrio sp. counts in the shrimp gastrointestinal (GI) tract. Five shrimp for enzymatic activity assays were also collected at the end of week 8 (see below) and the GI tract of each shrimp was removed aseptically and then immediately packed and immersed in liquid nitrogen. Samples were kept in -80°C until analysis. Similarly, two shrimp from each replicate were randomly collected to determine the expression levels of immune-related genes, such as prophenoloxidase (proPO), peroxinectin (PE), lipopolysaccharide- and β-1,3-glucan- binding protein (LGBP) and serine protein (SP)
In order to estimate Bacillus sp. and Vibrio sp. counts in the shrimp GI tract and rearing water, selective culture media were used as previously described. Shrimp were aseptically dissected using a sterile surgical scissor and the GI tract was removed and homogenized in a sterile glass homogenizer with PBS. To ensure the colonization of B. subtilis in shrimp GI tract, at the end of week 8, DNA of 10 random colonies from the plates within the acceptable range of colony counts (30e300 CFU plate -1 ) were extracted and identified by PCR according to conditions previously described.
2.5. Enzymatic activity analysis
The crude extract of the GI tract was used to quantify the digestive enzymatic activity of shrimp in different treatments. The whole of the GI tract of one or two shrimp were dissected, pooled, weighed and homogenized with cold deionized water (1:10). The homogenate was then centrifuged at 5000 g for 20 min at 4°C. The supernatant was carefully separated and passed through 0.45-mm pore-size filters (Sartorius, Germany). Aliquots were made in 1.5-ml Eppendorf tubes in triplicates and kept at -20°C to analyze different enzymes.
The total protein was measured using bovine serum albumin as standard according to Bradford. Total protease activity was assayed using casein as the substrate which reacts with Folin’s reagent. A calibration curve of absorbance at 440 nm was prepared using tyrosine as standard. One unit of protease activity was defined as the number of micromoles of tyrosine released per min per mg of protein at 37°C . Total amylase activity was determined according to Rick and Stegbauer, using 1% soluble starch as substrate reacting with 3,5-dinitrosalicylic acid. A calibration curve of absorbance at 550 nm was prepared using a standard maltose solution. One unit of amylase activity was defined as the number of micromoles of maltose released per min per mg of protein at 37°C.
2.6. Experimental infection
After eight weeks of the addition of probiotics into the rearing water, an experimental infection was induced in shrimp with the pathogenic bacterium, V. harveyi ATCC 14126. This strain was grown overnight in LB medium and the concentration was adjusted to 107 CFU ml-1 using NSS as above mentioned. A total of 30 shrimp in the intermolt stage were collected from the treatment and control groups and injected with 20 µl of the bacterial suspension into the third abdominal segment. Immediately after injection, shrimp were transferred into the 20 l tanks with 10 shrimp each. The experiment was conducted in triplicate and the water was supplied from the previous tanks in order to minimize the stress. A group of untreated shrimp with B. subtilis strains, which was injected with NNS, served as positive control (NB). During the experimental infection, shrimp were fed three times daily (8:00 am, 16:00 pm, and 24:00 pm). The mortality was monitored daily for up to 10 d.
2.7. Relative mRNA expression of immune-related genes
The expression of immune-related genes in shrimp induced by B. subtilis strains during V. harveyi infection was determined by realtime RT-PCR (RT-qPCR). Two shrimp from each replicate was randomly collected for RNA extraction. Since it was almost impossible to collect hemocytes from the shrimp (3.0 – 4.0 g), the whole body of shrimp was therefore freeze-dried using ample amounts of liquid nitrogen and homogenized using RNase-free mortar and pestle. An amount of 100 mg of this homogenate was used for RNA extraction and purification using the guanidinium thiocyanate method. Reverse transcription was used to synthesize the first-strand cDNA using QuantiTect Reverse Transcription Kit (Qiagen) containing the oligo-(dT)18. The manufacturer’s recommendations were followed to maximize cDNA synthesis. RT-qPCR assays were conducted with the following primers to determine expression levels of immune-related genes: for ProPO, forward (50-GCC TTG GCA ACG CTT TCA-30) and reverse (50-CGC GCA TCA GTT CAG TTT GT-30); for PE, forward (50-TGG ACC TCG CGG GAG AT-30) and reverse (50-GAC CGA TAG CCA CCA TGC TT- 30); for LGBP, forward (50-CAT GTC CAA CTT CGC TTT CAGA-30) and reverse (50-ATC ACC GCG TGG CAT CTT-30); for SP, forward (50-CGT CGT TAG GTT AAG TGC GTT CT-30) and reverse (50-TTT CAG CGC ATT AAG ACG TGTT-3R0). Expression levels were normalized using bactin (forward 50-GAG CAA CAC GGA GTT CGT TGT-30 and reverse 50- CAT CAC CAA CTG GGA CGA CAT GGA-30) as the housekeeping gene, following conditions previously described.
2.8. Statistical analysis
Data on growth parameters, digestive enzymatic activity, bacteriological analysis, immune response and disease resistance among treatments were analyzed by using ANOVA and Duncan’s multiple range test was used to determine the significant variation (P < 0.05). All statistical analysis was performed using SPSS, version 15 (SPSS Inc, Chicago, IL, USA).
3/ Results
3.1. In vitro assays on the ion concentrations
The ability of B. subtilis L10 and G1 to reduce ammonia, nitrite and nitrate ions was evaluated using synthetic water over a course of 24 h (Table 1). Both strains showed similar abilities to reduce ammonia and nitrite levels with rates of 0.044 and 0.53 mg l-1 h-1 , respectively. Different ability of nitrate reduction was recorded for L10 (2.43 mg l-1 h-1 ) and G1 (2.79 mg l-1 h-1 ). In addition, both strains showed the same growth pattern in the synthetic pond water. The highest density was recorded at 16 h for both strains and reduction of bacterial population was observed at 24 h (Table 1).
3.2. In vivo assays on water quality
Significant differences (P < 0.05) were observed in ammonia concentration among the probiotics and control groups starting from week 5 until the end of the experiment (Fig. 1). These differences ranged from 0.22 ± 0.08 to 0.78 ± 0.2 mg l-1 and 0.17 ± 0.2 to 0.66 ± 0.35 mg l-1 in the BM8 and BM5 groups, respectively, whereas the control group reached values from 1.04 ± 0.2 mg l-1 to the high concentration of 1.58 ± 0.45 mg l-1 at the end of the experiment. There were no significant differences (P > 0.05) in the nitrite concentration among all experimental groups, from the start of the experiment to week 5; however, significant differences (P < 0.05) in the nitrite concentration were observed in the BM8 group from week 6 to the end of the experiment, as compared to the BM5 and control groups. A drastic reduction in nitrite concentration was detected in the BM8 group, ranging from 0.007 ± 0008 to 0.0007 ± 0.0015 mg l-1 , whereas slight increases were observed in the BM5 and control groups, ranging from 0.0097 ± 0.0017 to 0.012 ± 0.001 mg l-1 and 0.010 ± 0.002 to 0.125 ± 0.001 mg l-1 , respectively (Fig. 1). The nitrate concentrations were significantly different (P < 0.05) only on week 7 and 8. The BM8 group showed the highest concentrations (P < 0.05) from 3.72 ± 0.3 to 4 ± 0.18 mg l-1 followed by the control (2.72 ± 0.59 and 3.07 ± 0.35 mg l-1 ) and BM5 (1.1 ± 0.29 and 1.55 ± 0.31 mg l-1 ) groups (Fig. 1).
Table 1: Response of B. subtilis L10 and G1 to ion reduction in the synthetic seawater
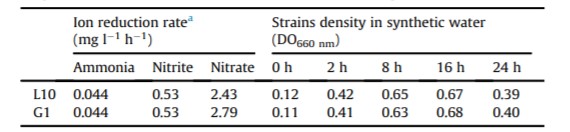
a: Ion reduction rate is calculated based on the 24 h incubation
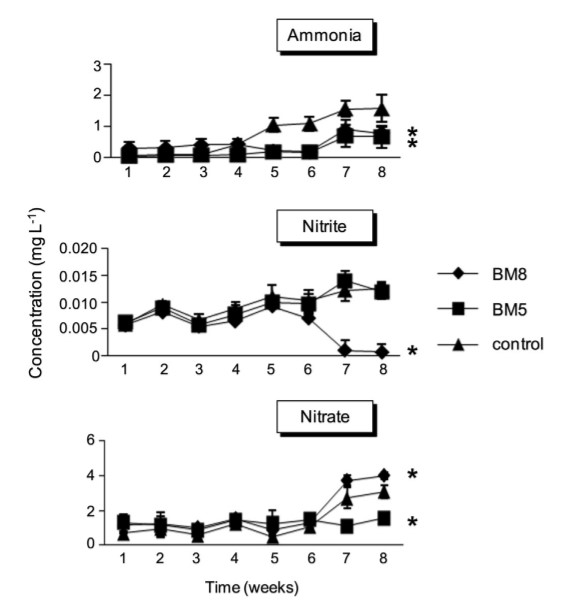
Fig. 1. Changes in concentration of residual ammonia, nitrite, and nitrate in rearing water of white shrimp treated with (A) BM8 (B. subtilis L10 and G1, 108 CFU ml-1 ); (-) BM5 (B. subtilis L10 and G1, 105 CFU ml-1 ); and (:) untreated shrimp as the control group. An asterisk denotes statistical significance, P < 0.05, compared to the control.
3.3. Growth parameters
There were no significant differences (P > 0.05) in the weight between the treatment and control groups at the start of the experiment. However, significant differences (P < 0.05) were observed in the final weight, weight gain, FCR and SGR of the shrimp between the treatment and control groups at the end of the experiment (Table 2). No significant difference (P > 0.05) was observed in the survival rate neither between the BM8 and BM5 groups nor between the BM5 and control groups; however, the survival rate of those shrimp in the BM8 group were significantly different (P < 0.05) from the control group (Table 2).
Table 2: Data of growth performance and survival of white shrimp treated with or without probiotics.
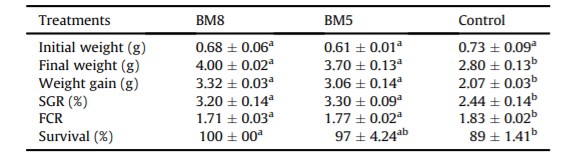
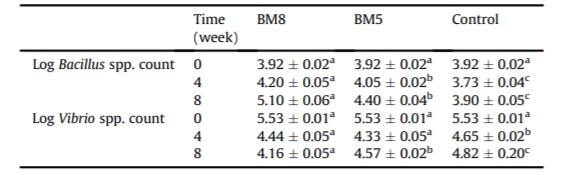
Table 3: Log mean of Bacillus spp. and Vibrio spp. count (CFU g-1 ) in the GI tract of white shrimp treated with or without probiotics for 55 days
3.4. Bacteriological analysis
Counts of Bacillus spp. and Vibrio spp. detected in the shrimp GI tract and rearing water at week 0, 4, and 8 are shown in Tables 3 and 4. There were significant differences (P < 0.05) for Bacillus spp. and Vibrio spp. counts in the shrimp GI tract between the treatments and control groups. At the end of the experiment, a significant increase (P < 0.05) of Bacillus spp. counts in the shrimp GI tract was observed in the BM8 and BM5 groups, ranging from 3.92 ± 0.02 to 5.1 ± 0.06 and 4.4 ± 0.04 log CFU g-1 , respectively. In contrast, low Bacillus spp. counts in the shrimp GI tract were found in the control group (Table 3). In order to confirm their taxonomical identity at species level, the yellow colonies counted on MYP agar as Bacillus spp. were randomly picked (n ¼ 10) and subjected for DNA amplification using specific primers. Bacteriological and molecular analyses showed the ability of B. subtilis L10 and G1 to colonize the shrimp GI tract. Moreover, a considerable reduction (P < 0.05) of Vibrio spp. counts in the shrimp GI tract was observed in the BM8 and BM5 groups at week 4 and 8, as compared to the control group (Table 3).
Counts of Bacillus spp. and Vibrio spp. detected in the rearing water between the treatment and control groups were significantly different during the experimental period. Bacillus spp. counts in the rearing water were significantly higher (P < 0.05) in the BM8 and BM5 groups as compared to the control group at week 4 and 8 (Table 4). Vibrio spp. counts were significantly higher (P < 0.05) in the control group than those in the BM8 and BM5 groups at week 4 and 8 (Table 4)
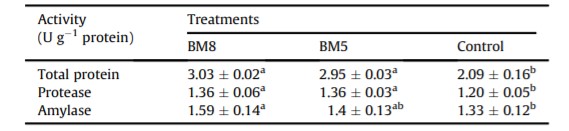
3.5. Total protein and enzymatic activity
Total protein, protease and amylase activity in the GI tract of shrimp were evaluated and statistically compared (Table 5). Significant differences in total protein and protease activity were observed in the shrimp GI tract of the BM8 and BM5 groups as compared to the control group (Table 5). Amylase activity was significantly greater in the shrimp GI of the BM8 group (1.59 ± 0.14 U g-1 protein) than in the control group; however, no difference was detected for amylase activity between the BM5 (1.4 ± 0.13 U g-1 protein) and control (1.33 ± 0.12 U g-1 protein) groups. The highest (P < 0.05) total protein and protease activity in the shrimp GI tract were found in the BM8 and BM5 groups, compared to the control group.
Table 4: Log mean of Bacillus spp. and Vibrio spp. count (CFU ml-1 ) in culture water of white shrimp treated with and without probiotics for 55 days.
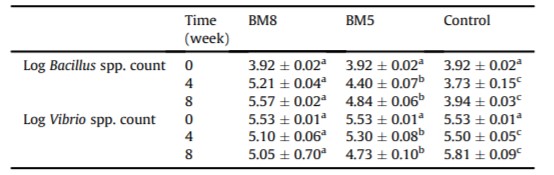
Table 5: Digestive enzyme activity in white shrimp treated with or without probiotics for 55 days.
3.6. Cumulative mortality and immune-related gene expression
After 8 weeks of the experiment, all experimental groups were challenged with V. harveyi (Fig. 2). After being injected with a high dose (106 CFU shrimp-1 ) of V. harveyi, significant differences (P < 0.05) in cumulative mortality were found in the MB8 (36.67 ± 5.77%) and MB5 (50 ± 10%) groups, compared to the control group (80 ± 10%). No mortality was observed in shrimp of the positive control, which had been injected with NSS.
The mRNA expression of four immune-related genes in all experimental groups was measured 24-h post-infection using RTqPCR assays. Significant differences for the up-regulation of proPO, PE, LGBP, and SP transcriptions were observed in shrimp from the BM5 group, as compared to the BM8 and control groups (Fig. 2). Moreover, LGBP expression was also significantly up-regulated in shrimp from the BM8 group, although to a lesser extent than the BM5 group, as compared to the control group (Fig. 3).
4/ Discussion
Previous studies have demonstrated the probiotic properties of several strains belonging to the genus Bacillus in some aquatic organisms. In the current study, two B. subtilis strains L10 and G1 showed a considerable reduction of ammonia, nitrite, and nitrate ions under in vitro conditions. Subsequently, the administration of these bacterial strains directly in the rearing water maintained the concentration of ammonia, nitrite and nitrate ions within the acceptable ranges for shrimp culture, particularly in the last three weeks of the experiment. Although these findings are in agreement with previous studies, Liu et al. showed that B. subtilis E20 did not contribute to the improvement of water quality for shrimp culture. Direct comparisons between these probiotic strains are, however, difficult as these strains are functionally different and may be differently affected by various factors such as genetics, nutrition and the environment.
This study also demonstrated that the application of B. subtilis strains in the rearing water had beneficial effects on the final weight, weight gain, SGR, FCR and survival of the treated shrimp compared to the control group. Previous studies have showed that the administration of probiotics as water additives can improve the growth performances and survival rate of shrimp species. Secretion of digestive enzymes in the GI tract could be another possible explanation for the better growth performance, which consequently results in optimal health and higher survival rate. Some studies have demonstrated that enzymes of the genus Bacillus are very efficient at breaking down a large variety of carbohydrates, lipids, and proteins into smaller units. In addition, administration of these B. subtilis strains increased total protein, protease and amylase activity of treated shrimp after the successful colonization in the GI tract. Thus, the colonization of these beneficial bacteria in the GI tract improved feed digestibility, which could result in more efficient growth rates
Competitive exclusion of probiotics is a key factor known for improving the microbial intestinal balance. This possible mode of action which leads the replacement of beneficial bacteria in the GI tract might subsequently contribute the growth performance and survival rate. In this study, administration of B. subtilis strains showed strong competitive exclusion and successful colonization in the GI tract of treated shrimp compared to the control group. These bacterial strains also conferred a higher resistance to V. harveyi, as compared to those shrimp without probiotic treatment. Similar results have been previously described. In fact, Balcázar & Rojas-Luna showed the effectiveness of dietary B. subtilis UTM 126 on the protection of white shrimp against vibriosis. Additionally, Zokaeifar et al.showed that dietary supplementation of B. subtilis led to a reduction in mortality after exposure to V. harveyi.
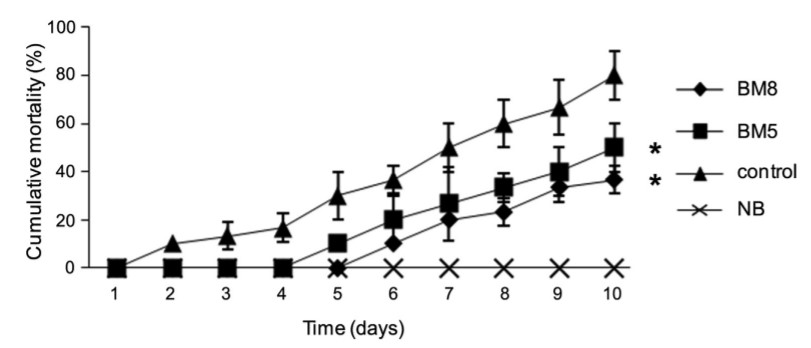
Fig. 2. Cumulative mortality (%) of white shrimp 10 days post-challenge with V. harveyi. Error bars represent the SD for each group at each time point. (A) BM8 (B. subtilis L10 and G1, 108 CFU ml-1 ); (-) BM5 (B. subtilis L10 and G1, 105 CFU ml-1 ); (x) NB (no addition of probiotics but injected with NSS); and (:) control (no addition of probiotics but injected with V. harveyi). An asterisk denotes statistical significance, P < 0.05, compared to the control.
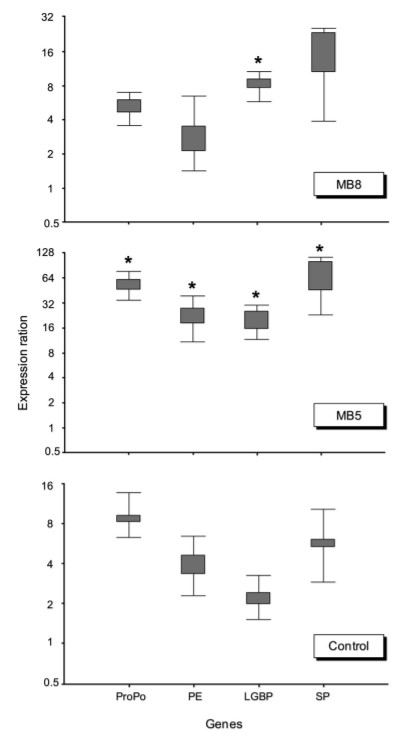
Fig. 3. The mRNA expression of four immune-related genes of white shrimp treated with or without probiotic bacteria for 55 days and challenged with V. harveyi. All analyses were performed using the REST software. Boxes represent the interquartile range, or the middle 50% of observations. Whiskers represent the minimum and maximum observations. An asterisk denotes statistical significance, P < 0.05, compared to the control.
To evaluate whether probiotic administration had an effect on the expression of immune-related genes, both B. subtilis strains were administered at two different doses, BM5 and BM8, in the rearing water and results showed significant differences for the upregulation of proPO, PE (the cell adhesive protein), LGBP (the recognition protein) and SP (key enzyme related to PO activity and activation) transcriptions in the shrimp of the BM5 group, as compared to the BM8 and control groups. Our results also demonstrated that LGBP expression in the BM8 group was significantly higher than the control group. Activation of the proPO system is through recognition molecules in the hemolymph of invertebrates, which is able to recognize and respond to intruders via lipopolysaccharides or peptidoglycan from bacteria and β-1,3-glucans from fungi. The binding of LGBP to lipopolysaccharides or β-1,3-glucans has been documented to activate the proPO system of crustaceans. These findings, therefore, indicate that immune response was partially responsible for mediating disease resistance.
Although both treatment, BM5 and BM8, conferred beneficial effects for shrimp culture, the dose at 108 CFU ml-1 (BM8) in the rearing water showed good results in terms of water quality, growth performance, digestive enzymatic activity, competitive exclusion, immune response and disease resistance.
By: Hadi Zokaeifar, Nahid Babaei, Che Roos Saad, Mohd Salleh Kamarudin, Kamaruzaman Sijam và Jose Luis Balcazar
Reference: http://dx.doi.org/10.1016/j.fsi.2013.10.007
“Domesticated Shrimp Postlarvae – The Key To Success”
See more:
- Differing Water Salinities Can Shift Bacterial Composition In Ras Shrimp Production
- Black Tiger Shrimp Processing Waste Can Be Converted Into A Value-Added Powder
- Global Shrimp Production To Surpass 5 Million MT in 2022, CP Foods’ Robin McIntosh Predicts

 Tiếng Việt
Tiếng Việt 中文 (中国)
中文 (中国)