Triggers and co-infections, symptoms and prevention measures for the opportunistic and ubiquitous Vibrio spp.
Vibriosis is a bacterial disease caused by Vibrio spp. These bacteria form part of the natural microbiota of wild and cultured shrimp and the marine environments. Vibrio infections have become a major constraint on the production and trade in shrimp aquaculture. They are responsible for several diseases and mortalities of up to 100% and cause crop losses globally. Vibrio-related infections frequently occur in hatcheries, but epizootics also commonly occur in grow-out ponds.
Pathogenic strains
Vibrio species such as Vibrio harveyi and Vibrio parahaemolyticus have been identified as primary pathogens in shrimp farming. Other species of Vibrio such as Vibrio vulnificus, Vibrio anguillarum, Vibrio campbellii and Vibrio splendidus have been associated with shrimp disease and caused massive epidemics.
Isolation
Thiosulfate-citrate-bile salts-sucrose agar (TCBS agar), is a type of selective agar culture plate that is used in microbiology laboratories to isolate Vibrio species. TCBS agar is highly selective for the isolation of Vibrio species and is easy to use in a farm laboratory. Furthermore, farmers are able to differentiate Vibrio species, depending on the colony colour: green and yellow. Alternatively, for V. parahaemolyticus enumeration, the use of chromogenic agar is also suggested.
Shrimp diseases associated with Vibrio spp.
Vibrio can remain in the environment without causing disease, but can very easily switch from opportunistic and commensal, to pathogenic when conditions change. Thus, its ability to cause disease, or increase in virulence, is a complex process affected by many variables, including host, Vibrio species/strain, developmental stage, physiological conditions, environmental stress and infection method.
There are several diseases associated with Vibrios. V. harveyi is one of the most important etiologic agents of luminous disease and causes mass mortalities in penaeid larval rearing systems. V. parahaemolyticus is responsible for the outbreaks of early mortality syndrome (EMS) or acute hepatopancreatic necrosis disease (AHPND). In the case of white faeces disease (WFD), Vibrio spp. have been isolated from WFD infected animals and a challenge using Vibrio isolates resulted in identical WFD in field specimens (Tran, 2019).

Figure 1. White faeces on shrimp pond (A) and (B).
Clinical signs of Vibrio infections include lethargy, loss of appetite, discoloured and necrotic hepatopancreas with the presence of “clumping”, red discolouration of the body, yellowing of the gill tissue, white patches in the abdominal muscle, melanisation, granulomatous encapsulation, necrosis and inflammation of organs (lymphoid organ, gills, heart) and luminescence.
Triggers and co-infections
Disease outbreaks caused by Vibrios may occur when environmental factors trigger the rapid multiplication of bacteria (which are already present at low levels). Vibrios become opportunistic pathogens when natural defence mechanisms are suppressed or exposed to stressful conditions.
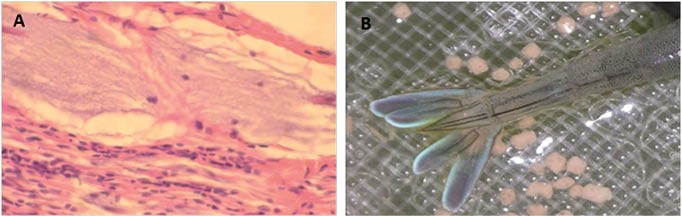
Figure 2. Necrosis on the muscular fiber caused by colonies of V. parahaemolyticus (A) and L. vannamei with greenish fluorescence on tail (B) (Courtesy Dariano Krummenauer)
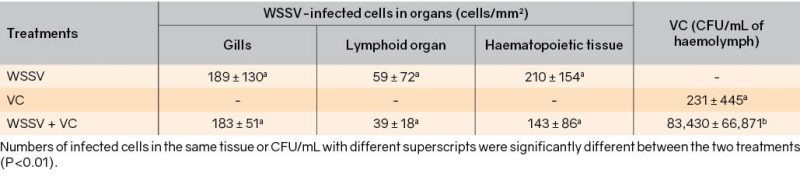
Vibrio spp. are also associated with multiple etiological agents. The rapid multiplication of Vibrios could be triggered by primary disease. Phuoc et al. (2009) reported on the rapid multiplication of V. campbellii in co-infection with white spot syndrome virus (WSSV, Table 1).
Vibrio threshold in shrimp farms
There are many Vibrio maximum threshold standards in farms. Most of them are for Vibrio spp. in pond water. These are the common maximum thresholds used by shrimp farms:
- Total Vibrio Count (TVC): varies from 103 CFU/mL to 104 CFU/mL. Some farmers adopt the TVC of 102 CFU/mL as the maximum threshold.
- Vibrio colonies: 102 CFU/mL for green and 103 CFU/mL for yellow colonies.
- Vibrio percentage: 5% to 10% TVC of total plate count, and 10% Vibrio green colonies of TVC.
The threshold variation depends on farm experiences, since different farms have different environmental challenges and characteristics.
Alfiansah et al. (2018) reported the Vibrio profile in intensive shrimp ponds using zero water exchange (Table 2). Vibrio profiles were recorded in pond water every 10 days, from day 10 to 70. The lowest level was found in day 10 with 102 CFU/mL. The Vibrio concentration increased to 103 CFU/mL in day 20, 30, 40, and 50. In day 60 and 70, the Vibrio level was 104 CFU/mL.
In a 2019 Biomin field trial, the Vibrio spp. profile in pond water was recorded weekly in five ponds. Initial sampling was on day 15 (15 days after shrimp stocking) and finished at day 104 (Figure 4). In this trial, the range of total Vibrio count (TVC) was 1.334 x 103 CFU/ mL to 6.793 x 103 CFU/mL. Green colonies were in the range of 0 CFU/mL to 2.570 x 102 CFU/mL. Compared to total plate count (TPC), the TVC ranged from 0.98% to 11.4%.
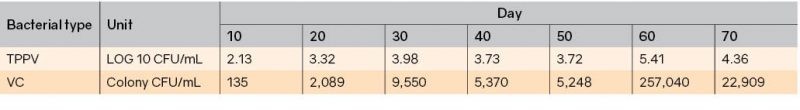
Table 2. Profile of TPPV (total potential pathogenic Vibrio) in intensive pond culture (Alfiansah et al. 2018)
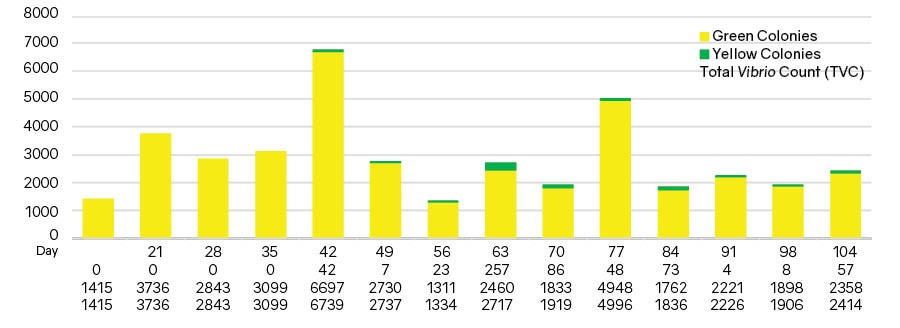
Figure 3. The average Vibrio green colonies, yellow colonies and TVC in normal shrimp pond
Shrimp farmers have been advised to monitor the Vibrio population in the shrimp gut and hepatopancreas. A study conducted by Rodriguez et al. (2015) can be used as a guide for the hepatopancreas. In this study, the reported Vibrio population in normal, initial and acute phase of AHPND in the hepatopancreas were 5.93 x 105 CFU/g, 1.78 x 106 CFU/g, and 1.65 x 108 CFU/mL respectively.
In the shrimp gut, it is common to find Vibrio populations at 106 CFU/g. In a Biomin trial the Vibrio population in shrimp gut was monitored every 5 days, from day 5 to 60. The Vibrio populations were 10 – 102 CFU/g on day 5 to 25. In day 30 to 60, the Vibrio population increased to 103-104 CFU/g. Farmers were advised to use 105 CFU/g as a maximum threshold of Vibrio in the shrimp gut. When this increases to 106 CFU/g, the shrimp will be at risk.
Management strategies for shrimp disease prevention and control
Vibrios are difficult to eradicate because they adapt well to different environmental conditions and can adopt a dormant state when facing adverse conditions. Pond management and robust gut health are important strategies to control Vibrio, together with frequent sampling to monitor their levels in the shrimp gut and pond ecosystem.
There are several strategies to prevent diseases caused by Vibrios: control the Vibrio population and mitigate the disease impact. Since the use of antibiotics to control these agents has led to problems of drug resistance and resulted in trade restrictions in export markets, the shrimp aquaculture industry continues to find more effective and environmentally friendly approaches in controlling Vibrio, such as biosecurity; disinfection, use of natural antimicrobials and probiotics.
Regarding disease mitigation, there are several strategies such as improving shrimp health or the immune system of the shrimp, a quorum quenching strategy, bind the toxin excreted by the Vibrio, and applying a complete biosecurity system. However, in this article we will only discuss the first strategy i.e. how to control Vibrio levels.
Biosecurity and disinfection
Disinfection, filtration and sanitation are examples of biosecurity practices. Disinfectants may be used to treat incoming water in the hatchery and grow-out phases. Chlorine, potassium permanganate, hydrogen peroxide, benzalkonium chloride, potassium monopersulfate, ozone and UV are popular disinfectants in shrimp farming. Each of these have different characteristics.
Chlorine is the most common disinfectant used in shrimp farming. The efficacy of chlorine is affected by pH, organic substance and biofilm formation. A study was conducted to evaluate the efficacy of sodium hypochlorite. In sterile seawater, at 1 and 5ppm levels, it was found that luminous bacterial V. harveyi populations were completely eliminated, from 106 CFU/mL in 30 minutes. However, in combination with an organic substance (0.1% peptone), it was demonstrated that chlorine was ineffective at inhibiting V. harveyi at inclusion rate less than 50ppm (Abraham et al., 2002). As a reminder, there have been reported cases of rapid increases in Vibrio numbers after the chlorine residues have disappeared. This is to be expected as chlorine will not only lower the number of Vibrio competitors but also kill algae, thus increasing nutrients for Vibrios to propagate.
Antimicrobials
Strategies to use antimicrobials to reduce the effects of the Vibrio (particularly V. parahaemolyticus) in the shrimp digestive system can help protect the shrimp. Certain essential oil (EO) mixtures and organic acid (OA) mixtures have been shown to be effective because of their inhibitory properties against Vibrio as shown in Figure 5. These compounds can be added to feed to be effective in the digestive system of the animal. The OA mixture has been demonstrated to possess an inhibitory effect on V. parahaemolyticus growth at doses of ≥5000 ppm and the EO mixture inhibited V. parahaemolyticus growth at doses of ≥500 ppm.
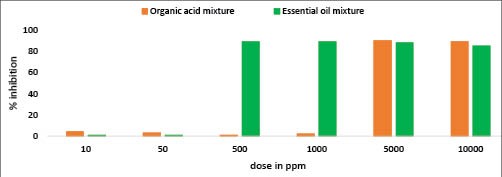
Figure 4. Growth inhibition of virulent V. parahaemolyticus after exposure to organic acid mixture and essential oil mixture. (Source: BIOMIN)
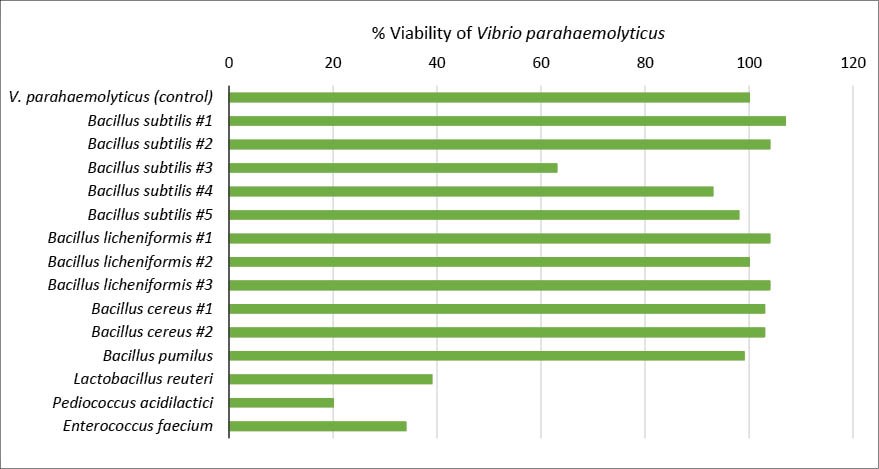
Figure 5. Varying effectiveness of probiotic bacteria against pathogenic V. parahaemolyticus. (Source: BIOMIN)
Probiotics
Probiotics is another means of disease control which have been useful in aquaculture. Probiotics are administered directly in water or via feeds. The mode of action of the probiotics is to actively inhibit the colonisation of potential pathogens in the digestive tract by the production of bactericidal substances, competition for nutrients and space, and modulation of the immune system. With carefully formulated probiotics, the modes of action may be complementary and/or synergistic. For example, providing greater disease resistance by improving the immune response and pathogen exclusion.
A study was conducted to analyse probiotic species in inhibiting the growth of the pathogenic V. parahaemolyticus. As shown in Figure 6, the probiotic strains such as Pediococcus acidilactici, Enterococcus faecium, Lactobacillus reuteri and Bacillus subtilis #3 (proprietary Biomin probiotics) were shown to inhibit V. parahaemolyticus. This study shows that not every menace can be targeted with Bacillus bacteria.
By Aqua Culture Asia Pacific
Reference: https://aquaasiapac.com/2020/09/29/revisiting-vibriosis-in-shrimp-aquaculture/
DOMESTICATED SHRIMP POSTLARVAE – THE KEY TO SUCCESS
See more:
- Can this breeding technique breathe new life into giant freshwater prawn farming?
- How much is genetics really improving the health and growth rates of farmed vannamei shrimp?
- The advantages of employing a shrimp nursery system

 Tiếng Việt
Tiếng Việt 中文 (中国)
中文 (中国)